6.2.5 Sketch and explain qualitatively the Maxwell–Boltzman energy distribution curve for a fixed amount of gas at different temperatures and its consequences for changes in reaction rate.
This graph shows the rate of reaction depends on the proportion of particles that have values of kinetic energy greater than activation energy. –The red line shows that the kinetic energy of the particle is lower, so the green shaded area has more particles than the blue area at the end of the reaction.
Maxwell-Boltzman Distribution
The distribution of the kinetic energy is shown by the Maxwell-Boltzman Distribution. The speed of the particles’ movement show the amount of kinetic energy they possess. The greater the speed of the particles shows they have more kinetic energy. The total of particles remains constant, despite the temperature increasing the area under the curve. The higher velocity the particles move show the higher average kinetic energy.
6.2.6 Describe the effect of a catalyst on a chemical reaction.
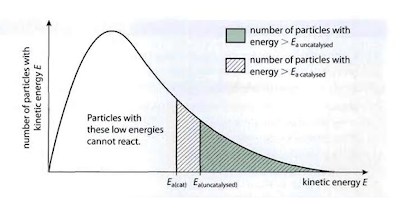
A catalyst “is a substance that increases the rate of reaction without itself undergoing permanent damage” –It provides the reaction an alternative route that has a lower activation energy.
In a reaction with a catalyst, without increasing the temperature, a larger number of particles has the value of kinetic energy greater than the activation energy and so will be able to undergo successful collisions.
6.2.7 Sketch and explain Maxwell– Boltzmann curves for reactions with and without catalysts.
Without a catalyst: there are less particles that has the value of kinetic energy greater than the activation energy. Therefore, the rate of reaction will be slower since there are fewer number of particles that will undergo successful collisions.
With a catalyst: there are more particles that have the value of kinetic energy than the activation energy. [activation energy is lower] So the rate of reaction will be faster as a greater number of particles will be able to undergo successful collisions.



Thanks Nathalie - please upload the questions and answers to the IB questions we did in class yesterday to help with your revision
ReplyDelete